The comparison of any physical quantity with its standard unit is called Measurement.
Physical Quantities
All the quantities which can be measured directly or indirectly in terms of which the laws of Physics are described are called physical quantities.

Units
A standard amount of a physical quantity chosen to measure the physical quantity of the same kind is called a physical unit. It should be easily reproducible, internationally accepted.
Fundamental Quantities and Their Units
Those physical quantities which are independent to each other are called fundamental quantities and their units are called fundamental units.
| Fundamental Quantitites | Name | Symbol |
| Length | Metre | m |
| Mass | Kilogram | kg |
| Time | Second | s |
| Electric Current | Ampere | A |
| Thermodynamic temp. | Kelvin | K |
| Amount of substance | Mole | mol |
| Luminous intensity | Candela | cd |
Supplementary Fundamental Quantities
Radian and steradian are two supplementary fundamental units. It measures plane angle and solid angle respectively.
| Supplementary fundamental quantities | Name | Symbol |
| Plane angle | Radian | rad |
| Solid angle | Steradian | sr |
Derived Quantities and Their Units
Those physical quantities which are derived from fundamental quantities are called derived quantities and their units are called derived units e.g. Velocity, acceleration, force, work etc.
System of Units
A system of units is the complete set of units, both fundamental and derived, for all kinds of physical quantities. The common system of units which is used in mechanics are given below:
- CGS System: In this system, the unit of length is centimetre, the unit of mass is gram and the unit of time is second.
- FPS System: In this system, the unit of length is foot, the unit of mass is pound and the unit of time is second.
- MKS System: In this system, the unit of length is metre, the unit of mass is kilogram and the unit of time is second.
- SI System: The system of units, which is accepted Internationally for measurement is the System Internationaled units abbreviated as SI. This system contains Seven fundamental units and two supplementary fundamental units.
Relationship between Some Mechanical SI Unit and Commonly Used Units
| S.No | Physical Quantities | Unit |
| 1. | Length | (a) 1 micrometre = 10-6 m (b) 1 nanometre = 10-9 m (c) 1 angstrom = 10-10 m |
| 2. | Mass | (a) 1 metric ton = 103 kg (b) 1 pound = 0.4537 kg (c) 1 amu = 1.66 × 10-23 m |
| 3. | Volume | 1 litre = 10-3 m3 |
| 4. | Force | (a) 1 dyne = 10-5 N (b) 1kgf = 9.81 N |
| 5. | Pressure | (a) 1 kgf-m2 = 9.81 Nm-2 (b) 1 mm of = 133 Nm-2 (c) 1 Pascal = 1 Nm-2 (d) 1 atmosphere pressure = 76 cm of Hg = 1.01 × 105 Pascal |
| 6. | Work and Energy | (a) 1 erg = 10-7 J (b) 1kgf-m = 9.11 J (c) 1 kWh = 3.6 × 106 J (d) 1 eV = 1.6 × 10-19 J |
| 7. | Power | (a) 1 kgf-ms-1 = 9.81 W (b) 1 horse power = 746 W |
Some Practical Units of Length
- 1 fermi = 10-15 m
- 1 X-ray Unit = 10-13 m
- 1 astronomical unit = 1.49 × 1011 m
- 1 light year = 9.46 × 1015 m
- 1 parsec = 3.08 × 1016 m = 3.26 light year
Dimensions
Dimensions of any physical quantity are those powers to which the fundamental quantities are raised to express that quantity. The expression of a physical quantity in terms of its dimensions, is called its dimensional formula.
Dimensional Formula of Some Physical Quantities
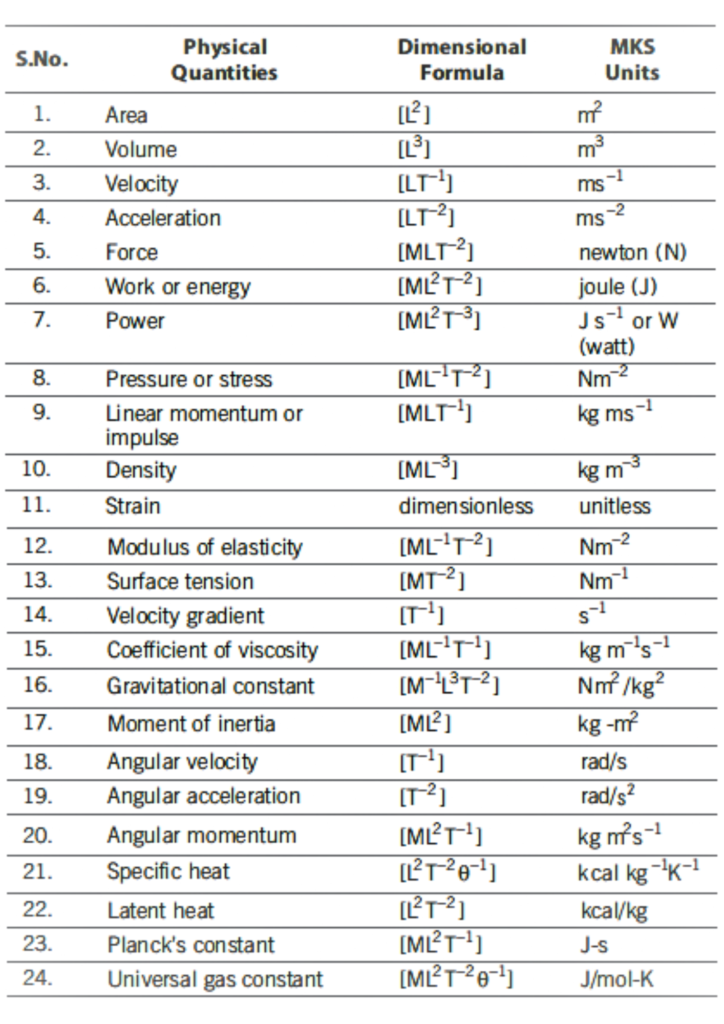
Homogeneity Principle
If the dimensions of left hand side of an equation are equal to thedimensions of right hand side of the equation, then the equation isdimensionally correct. This is known as homogeneity principle. Mathematically, [LHS] = [RHS].
Applications of Dimensions
- To check the accuracy of physical equations.
- To change a physical quantity from one system of units to another system of units.
- To obtain a relation between different physical quantities.
Significant Figures
In the measured value of a physical quantity, the number of digits about the correctness of which we are sure plus the next doubtful digit, are called the significant figures.
Rules for Finding Significant Figures
- All non-zeros digits are significant figures, e.g. 4362 m has 4 significant figures.
- All zeros occuring between non-zero digits are significant figures, e.g. 1005 has 4 significant figures.
- All zeros to the right of the last non-zero digit are not significant, e.g. 6250 has only 3 significant figures.
- In a digit less than one, all zeros to the right of the decimal point and to the left of a non-zero digit are not significant, e.g. 0.00325 has only 3 significant figures.
- All zeros to the right of a non-zero digit in the decimal part are significant, e.g. 1.4750 has 5 significant figures.
Significant Figures in Algebraic Operations
(1). In Addition or Subtraction: In addition or subtraction ofthe numerical values, the final result should retain as manydecimal places as there are in the number with the least places. e.g.
- If L1= 4.326 m and L2= 1.50 m
- Then, L1+ L2 = (4.326+1.50) m = 5.826 m
- As L2 has measured upto two decimal places, therefore L1+ L2 = 5.83 m
(2). In Multiplication or Division: In multiplication or division of the numerical values, the final result should retain as many significant figures as there are in the original number with the least significant figures. e.g. If length L = 12.5 m and breadth B = 4.125 m.
- Then, area A = L × B = 12.5 × 4.125 = 51.5625 m2
- As L has only 3 significant figures, therefore
- A = 51.6 m2
Rounding Off
The process of omitting the non significant digits and retaining only the desired number of significant-digits, incorporating the required modifications to the last significant digit is called rounding off the number.
Rules for Rounding Off a Measurement
- If the digit to be dropped is less than 5, then the preceding digit is left unchanged. e.g. 1.54 is rounded off to 1.5.
- If the digit to be dropped is greater than 5, then the preceding digit is raised by one. e.g. 2.49 is rounded off to 2.5.
- If the digit to be dropped is 5 followed by digit other than zero, then the preceding digit is raised by one. e.g. 3.55 is rounded off to 3.6.
- If the digit to be dropped is 5 or 5 followed by zeros, then the preceding digit is raised by one, if it is odd and left unchanged if it is even. e.g. 3.750 is rounded off to 3.8 and 4.650 is rounded off to 4.6.
Error
The lack in accuracy in the measurement due to the limit of accuracy of the measuring instrument or due to any other cause is called an error. The difference between the measured value and the true value of a quantity is known as the error in the measurement.
Errors are usually classified as:
(1). Absolute Error
The difference between the true value and the measured value of a quantity is called absolute error. If a a a1, a2, a3,…,an are the measured values of any quantity a in an experiment performed n times, then the arithmetic mean of these values is called the true value (am)
$$a_m=\frac{a_1+a_2+a_3+\dots+a_n}n$$
The absolute error in measured values is given by:
- Δa1 = am– a1
- Δa2 = am– a2
- Δan = am– an
(2). Mean Absolute Error
The arithmetic mean of the magnitude of absolute errors in all the measurement is called mean absolute error.
$$\overline{\triangle a}\;=\frac{\left|\bigtriangleup a_1\right|+\left|\bigtriangleup a_2\right|+…+\;\left|\bigtriangleup a_n\right|}n$$
(3). Relative Error or Fractional Error
The ratio of mean absolute error to the true value is called relative error.
$$Relative\;error\;=\;\frac{Mean\;absolute\;error}{True\;value}=\frac{\overline{\bigtriangleup a}}{a_m}$$
(4). Percentage Error
The relative error expressed in percentage is called percentage error.
$$Percentage\;error\;=\;\frac{\overline{\bigtriangleup a}}{a_m}\times100\%$$